A global medical device leader streamlined failure analysis and regulatory compliance.
Summary
- A global medical device leader streamlined failure analysis and maintained regulatory compliance with Lumafield's Neptune CT scanner.
- Lumafield empowered a 4,750% increase in annual scan volume while saving the customer over $335K in outsourced CT costs.
- Lumafield's validated hardware and software met stringent FDA requirements, ensuring seamless integration into quality processes.
- In-house CT slashed analysis time from three weeks to just a few minutes, enabling same-day defect resolution and protecting patient safety.
Background
Our customer is a multinational medical technology company known for developing medical devices, diagnostic systems, and pharmaceutical delivery solutions that serve billions of people globally. Their focus on safely improving patient outcomes has positioned them as a trusted partner to most top healthcare providers as well as pharmaceutical and biotech companies. This deep commitment to product quality is a central tenet of the company's global operations, which currently includes over 75K employees.
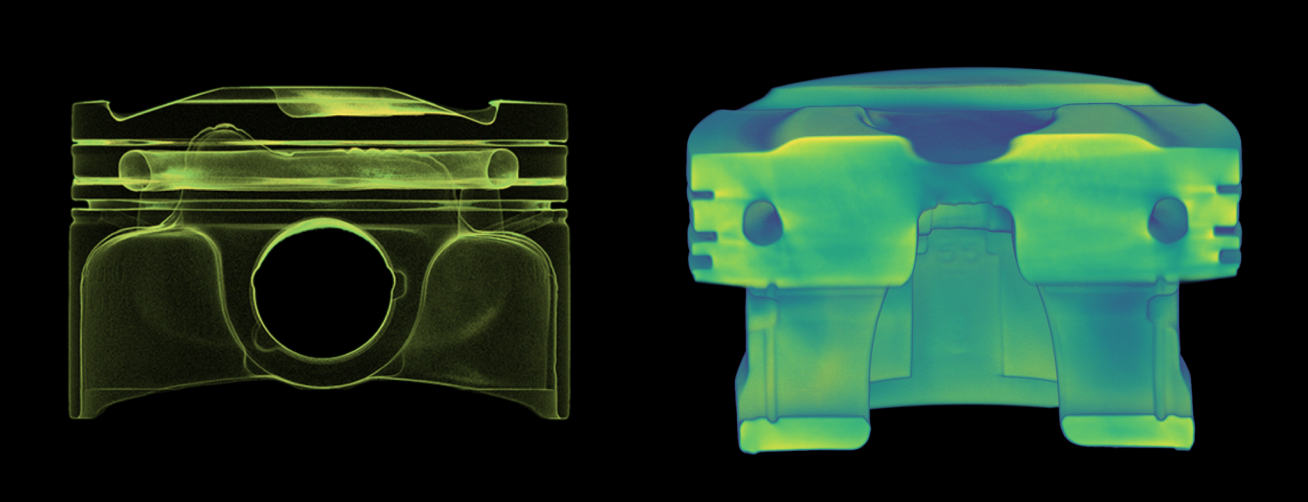
Some of the company’s most critical medical devices are intricate auto-injectors, pen injection systems, and needles for insulin delivery. These injector products are made from a combination of plastic components, springs, and a needle. The company’s 40+ step production process includes automated inspection at various stages and holds the parts to the highest standards of quality and consistency — a requirement of any company balancing FDA regulations and pharmaceutical industry standards.
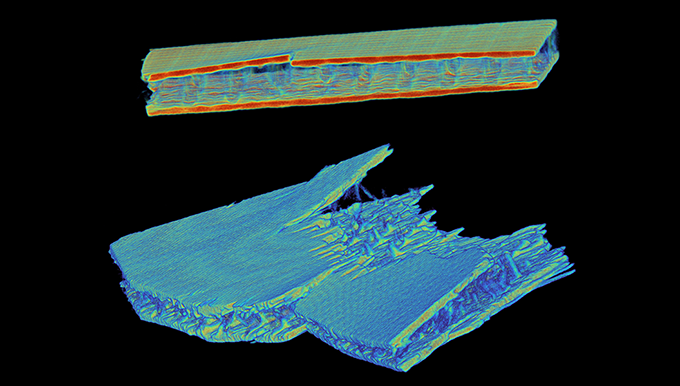
With over 1B units produced per year, defective products are inevitable, despite rigorous quality assurance standards greatly reducing the risk. When one of their auto-injectors doesn’t work as intended, the company’s engineers need to immediately understand what caused the failure.
A Validated Hardware Solution
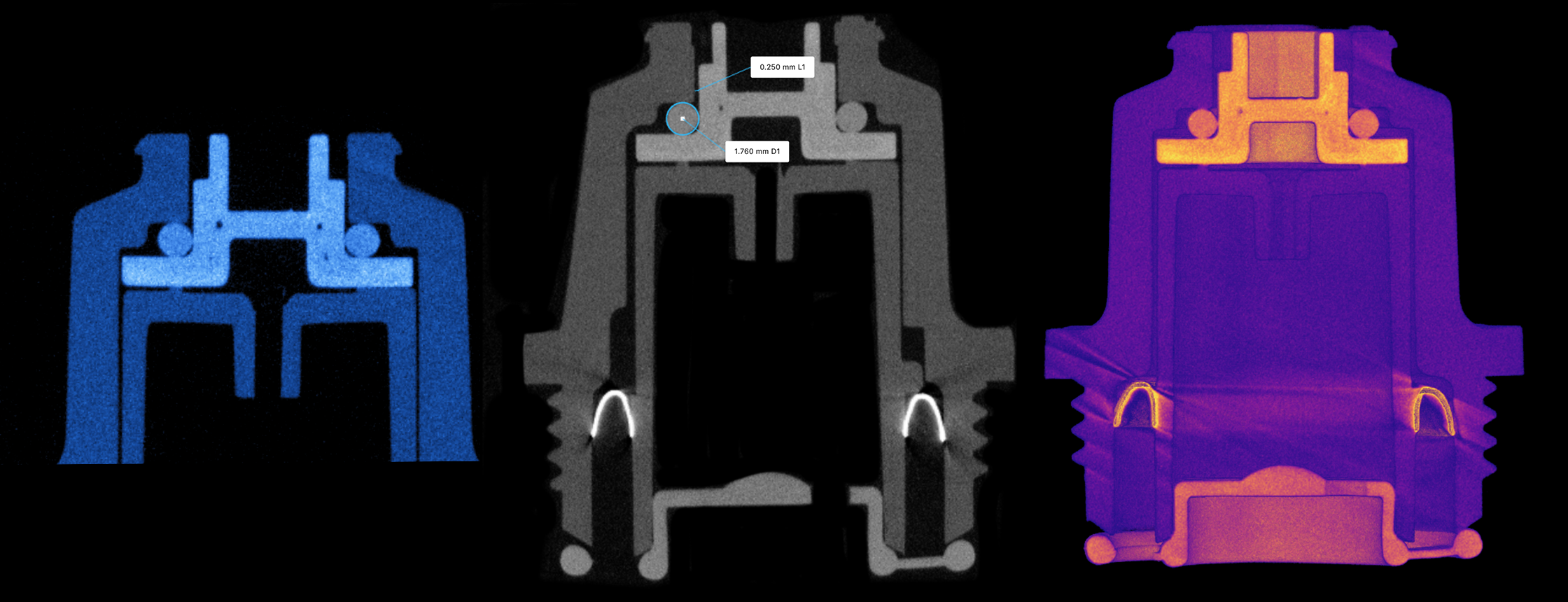
After years of outsourcing CT scans and using destructive methods to carry out failure analyses, our customer went in search of an in-house option. They needed a solution capable of meeting stringent FDA regulations, which include documented validation of the hardware and software, installation qualification and operational qualification (IQ/OQ) reports, and safety testing. These IQ/OQ reports ensure that all elements of a piece of equipment adhere to the manufacturer's design specifications and are installed and assembled correctly in the factory or plant.
Lumafield’s Neptune CT scanner was the best in-house option for the company’s engineering team from day one. Lumafield’s comprehensive deployment process — which includes on-site assembly, installation, and detailed reconstruction calibration — met the customer’s high standards for IQ/OQ reporting. Lumafield provides each of its customers with dedicated Solutions Engineers who conduct in-person training and offer around-the-clock support, continually optimizing scan strategies and customizing workflows to fully meet every customer’s inspection needs.
We wanted the best CT scanner on the market, but we realistically felt like we were going to have to settle for a company able to adhere to our validation and IQ/OQ standards… then we found Lumafield. The Neptune scanner delivered everything we were looking for and more.
— Director of Quality Control
Their work with the FDA and in the pharmaceutical industry also requires the company to ensure software validation compliance from any and all software programs they use. Lumafield’s cloud-based Voyager software delivered exactly that. Lumafield’s team provided the necessary validation documentation and continues to supply relevant information as needed with every software update to ensure ongoing regulatory compliance.

Maximizing Efficiency with In-House CT
With their new Neptune scanner up and running, the company immediately saw the impact they had been hoping for. Neptune cut their existing failure analysis CT scanning process from three weeks to just a few minutes. Without the need to ship samples across the country or worry about temperature-controlled packaging, the company’s engineers were able to focus their full attention on immediately resolving product defect issues.
Free from the financial burden of paying per scan, engineers conducted more than 4x as many scans in their first month using Neptune than they had the entire previous year. In their first year with Neptune in-house, they increased their annual scan volume by 4,750% and saved over $335K in outsourced CT scanning in the process.
Scanning at this volume was something we never could have afforded before we found Lumafield. It has completely transformed our understanding of defective products and allowed us to address defects the same day they arise. The impact has been incredible.
— Director of Quality Control