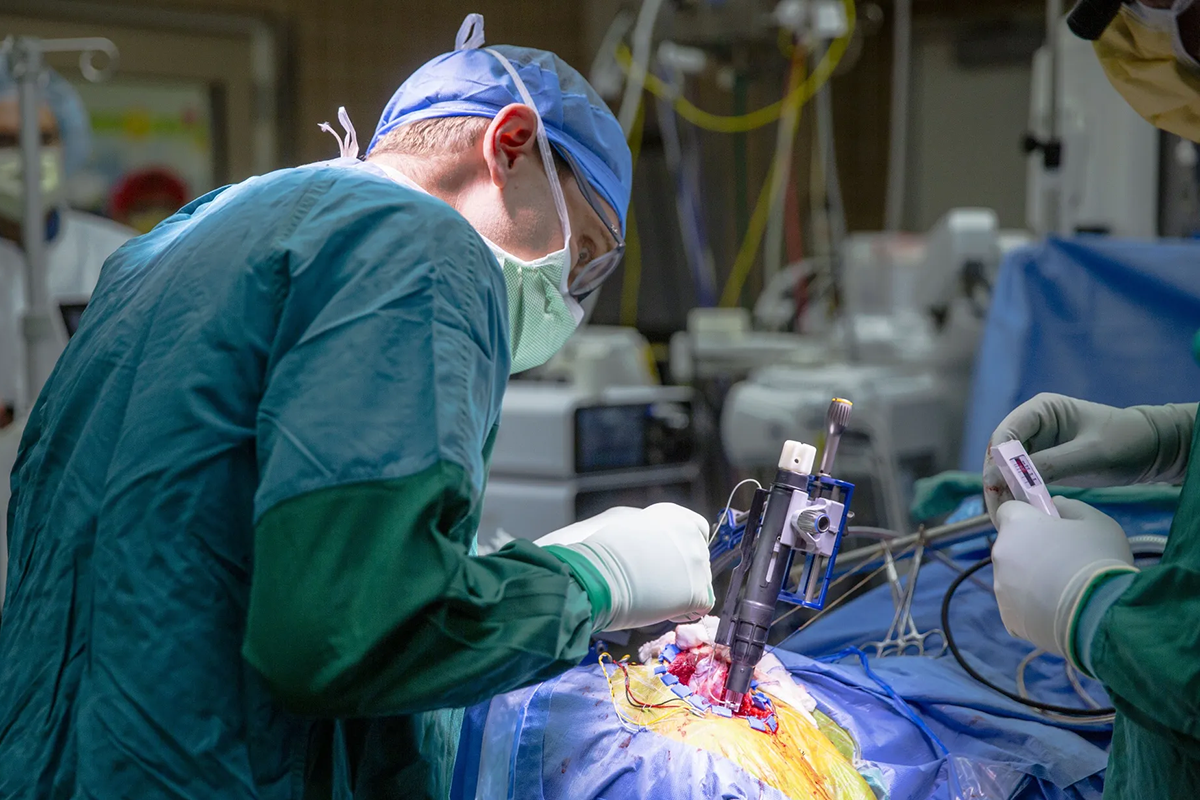
When you’re building an implanted medical device for the brain, you can’t cut corners. Engineering for performance is a given when you’re designing for a living system. To ensure that there are no adverse effects, an active implantable device cannot dissipate excessive heat, or there could be a risk to the surrounding tissue. Every decision, from materials, to power draw, and even packaging, is shaped by the need to do no harm. Designing a medical device is a totally different world from consumer electronics.
Stakes of the system
At Paradromics, we’re building a brain-computer interface (BCI) to restore speech for people with severe communication disorders: conditions like ALS or spinal cord injury that disrupts the connection between intention and expression. Our first product, Connexus® BCI, records directly from the brain’s speech centers and synthesizes intended speech in real time.

Our goal is a device that never has to be replaced. That means every material choice and assembly step matters.
My focus as an electrical engineer is on the Cortical Module itself. That’s the component that sits on the surface of the brain, and it’s also the point where the technical demands are highest. The Cortical Module must be reliable, power-efficient, and manufacturable at scale. And it has to last a long time; because we are not interested in devices that require users to have brain surgery to replace their brain implant every two years as with some technologies being explored by others. Our goal is a device that never has to be replaced. That means every material choice and assembly step matters.
People often compare implants like ours to wearable electronics, such as a smartwatch or continuous glucose monitor, as they share some similar goals: small size, clean data, minimal intrusion. But once you’re under the skin, everything changes. Now you’re dealing with strict FDA regulations, biocompatibility constraints, and failure modes with the highest possible stakes.
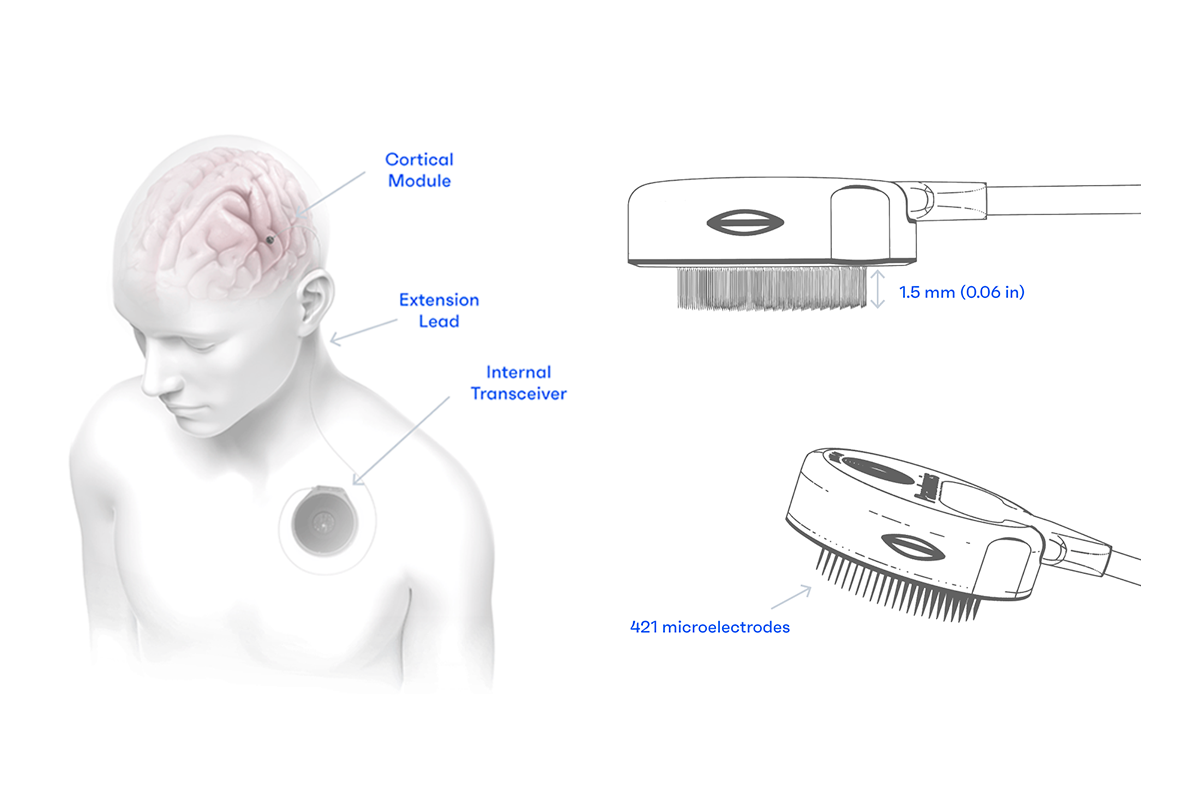
Incompressible timelines
Some timelines are just incompressible, and you have to come to terms with that. You can move fast in design, but once the project moves into manufacturing and validation, your pace becomes capped to that of regulation and biology. I wish someone had told me going into this field, that even with unlimited resources and engineering expertise, there are parts of the process you simply can’t speed up.
Regulatory requirements shape design from day one. Connexus BCI is classified as a Class III medical device, the same category as a pacemaker. Implanted medical devices are typically limited to known biocompatible materials, and to support a minimum of a 10 year implant life that means materials like titanium, platinum, and gold. You can try to get new materials approved, but it’s a much longer road. And when you’re already working on a novel device, it’s wiser to avoid adding extra unknowns.
Building the plane while flying it
There’s no off-the-shelf playbook for what we’re doing. Our system isn’t life-supporting like a pacemaker, but it is life-enhancing. We work closely with the FDA to define safety and benefit to the patient for a device that doesn’t quite fit their existing categories. We’re being evaluated, while also helping define the rules.
You have to keep moving forward, even while you’re clearing the path beneath your feet.
The metaphor we use internally is “building the plane while flying it.” It may sound chaotic, but it’s just the reality of working in a space this new. You have to keep moving forward, even while you’re clearing the path beneath your feet.
Testing for the future
That pressure to get it right shows up most in testing. Our devices go through a long chain of inspection, step by step. Some processes require equipment so specialized there’s no commercial equivalent, meaning we have to do it ourselves. That’s why we use Lumafield's industrial CT scanner to evaluate process outcomes and observe internal structures that can’t be seen any other way. It’s one of the few tools that can keep up with the level of precision we need.
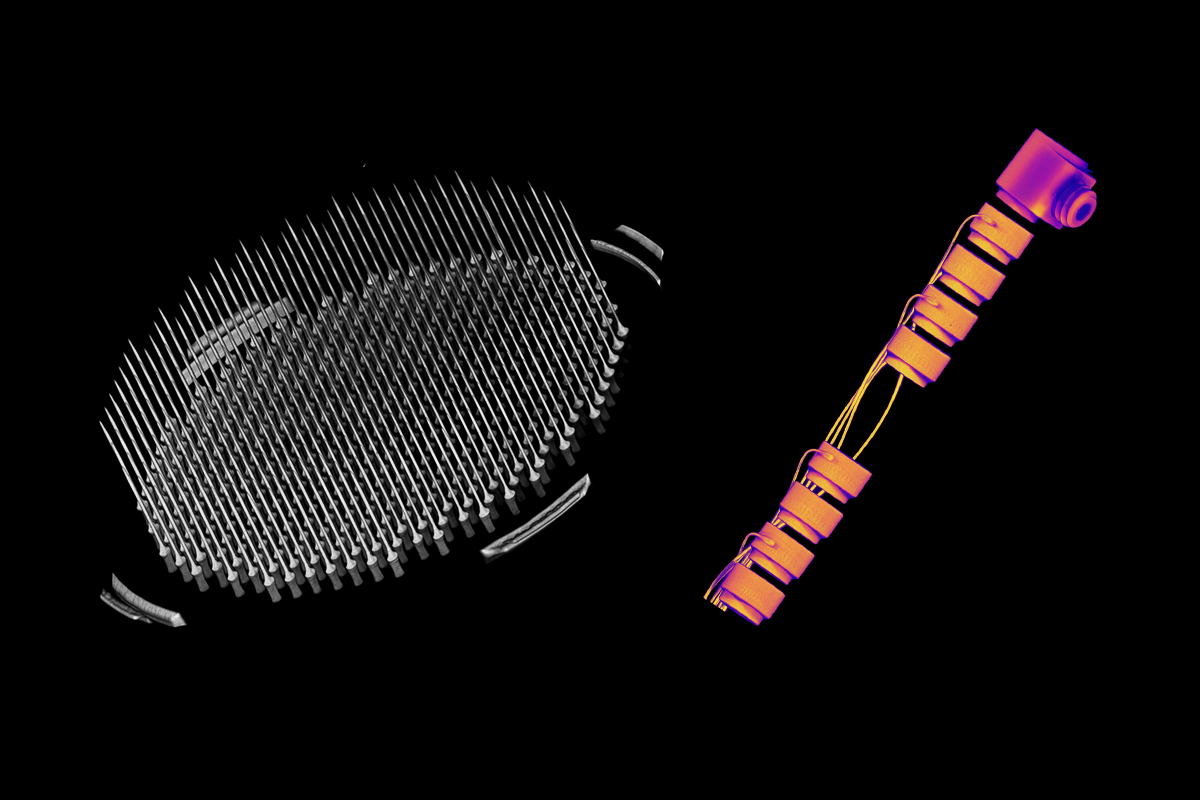
In addition to testing in a large animal model, we also run accelerated aging tests, environmental stress simulations, and strenuous mechanical validation to make sure our devices can survive in the body for years. This includes checking for things like thermal performance, electrode durability, and even the consistency of raw materials from one vendor to another.
Milestones that matter
This work isn’t theoretical. Earlier this year, we completed our first human implantation in a patient undergoing unrelated brain surgery. The successful recording and data collection demonstration confirmed that our device performs well in the real world. We saw the kind of neural activity we expected and it validated years of animal studies and lab work.
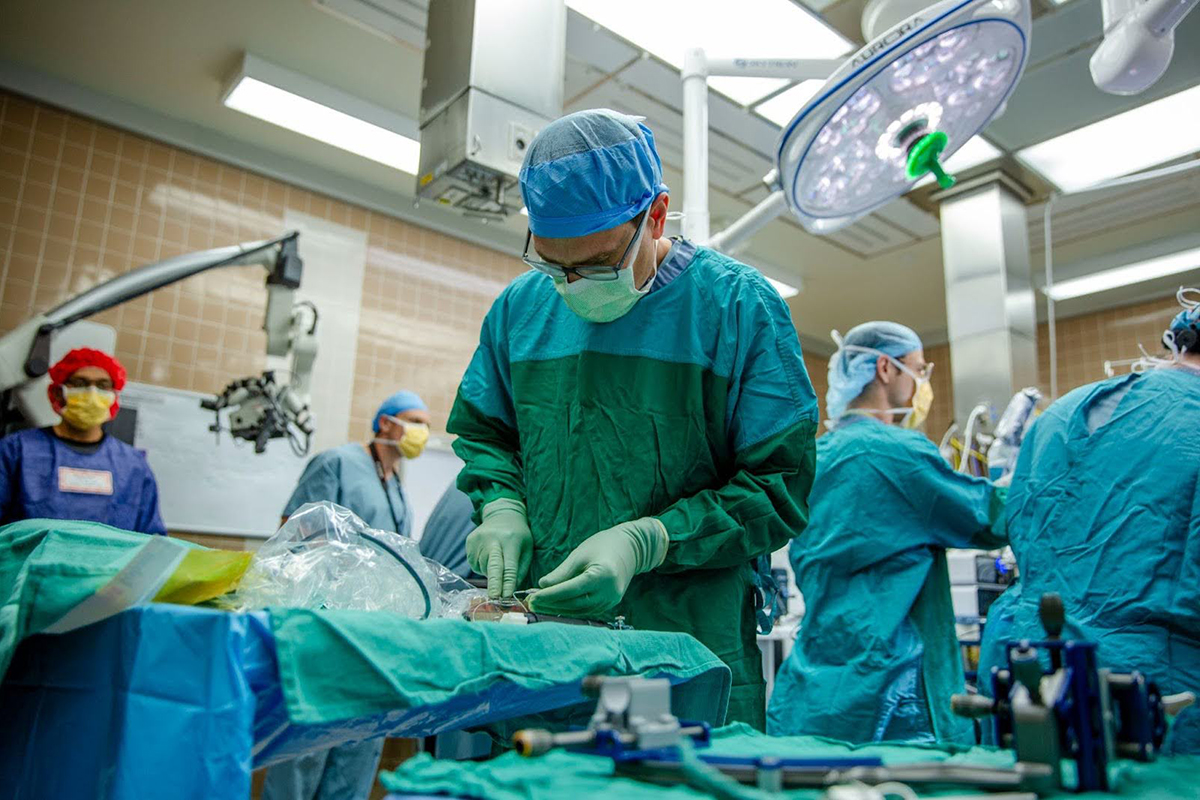
That milestone puts us on the path toward an Early Feasibility Study (EFS), a longer-term clinical investigation, where the device stays in place and is used regularly by a small number of participants. It’s the next big step toward FDA approval.
Designed to stay
I joined Paradromics right out of college in 2022, drawn to the cutting-edge intersection of engineering and neuroscience. It’s still a young field, and there’s so much left to figure out. But if we’re going to make a real difference for patients, we have to meet the body where it is. That means durability, reliability, and empathy for the people at the center of it all.
This Cortical Module should feel less like a medical device and more like a part of someone’s life. If we can get there, we’ll have done our job.